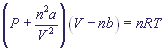 , where
, where
REAL GAS EQUATION
Real (imperfect) gas equation is called Van der Waals' equation. Real gases seldom follow the perfect gas law. Maximal deviation from the perfect behaviour occur at high pressure and low temperature values. Under these conditions the volume of the system becomes relatively small, and the occupied volume of molecules makes a remarkable part of the general volume. Besides, molecules are at near distances from one another, and this causes intermolecular interaction. Van der Waals suggested including two additional members into the real gas equation: constant a, to compensate decreasing of pressure caused by intermolecular attraction; and constant b, which presents effective volume of gas molecules:
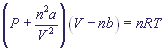 , where
, where
p - is pressure,
V - is volume,
n - is the amount of moles of the gas in the given volume,
R - is the universal gas constant,
T - is absolute temperature,
a, b are the van der Waals' constants, which are adjusted for different gases empirically according to the values of their departure from the ideal behaviour. They are calculated for 1 mole of gas. Their numerical values can be found in special charts.