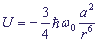 , where
, where
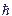 - is the Plank constant,
- is the Plank constant,
ω0 - is the frequency of the initial oscillation of the oscillators,
r - is the distance between the oscillators,
a - is polarizability of the oscillator.
DISPERSION INTERACTIONS
Dispersion interactions (London dispersion forces) are weak interactions out of the group of van der Waals bonds. They are interaction between two dipoles, which are electrical duplets. These interactions operate on very short distances. In non-polar molecule, in the absence of the field, electrons cannot be immovable, that is why the dipole moment is equal to zero only at the average. Dipoles oscillate inducing each other, and as the result attractive forces appear between them. Dispersion forces are of quantum-mechanical nature. The energy of such interaction is equal to:
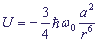 , where
, where
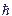 - is the Plank constant,
- is the Plank constant,
ω0 - is the frequency of the initial oscillation of the oscillators,
r - is the distance between the oscillators,
a - is polarizability of the oscillator.
Dispersion forces are important for interactions of atomic groups and molecules with saturated valence bonds