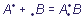 ), while in case of the donor-acceptor bond the pair is provided completely by one of fragments, participating in bonding (
), while in case of the donor-acceptor bond the pair is provided completely by one of fragments, participating in bonding (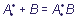 ).
).
DONOR-ACCEPTOR BOND
Donor-acceptor bond (coordination bond) is a chemical bond, which is realized in complex compounds, when atoms with free electron pairs (donors) tend to use them for bounding with other particles, while atoms without complete electronic configuration (acceptors) tend to fill up their outer electronic sheath by using somebody else's electron pairs. If both tendencies are strong enough, then a bond appears between the pairs due to an electron pair of the donor.
The nature of the donor-acceptor bond does not differ from the nature of a usual
polar covalent bond. The only difference is in the origin of connecting electron pair: the covalent bond is formed of unpaired electrons of each of the interacting atoms (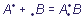 ), while in case of the donor-acceptor bond the pair is provided completely by one of fragments, participating in bonding (
), while in case of the donor-acceptor bond the pair is provided completely by one of fragments, participating in bonding (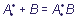 ).
).