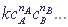 , where
, wherecA, cB, ... are concentrations of reacting substances A, B, ... ;
nA, nB, ... are the degrees of order of the reaction to the corresponding agents (as a rule, they coincide with the stoichiometric coefficients of these reagents in the chemical equation)
k is the coefficient of proportionality, the constant of the reaction rate, which depends on the nature of the reacting substances and on the temperature;
v is the reaction rate.
It is usually assumed that v =
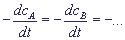 .
Then from the kinetic equation, by solution of the corresponding differential equation, dependence of v and
.
Then from the kinetic equation, by solution of the corresponding differential equation, dependence of v and 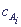 on time t, that is complete kinetic description of the
system, can be attained.
on time t, that is complete kinetic description of the
system, can be attained.